Our documents
Find the publications of the association’s working groups
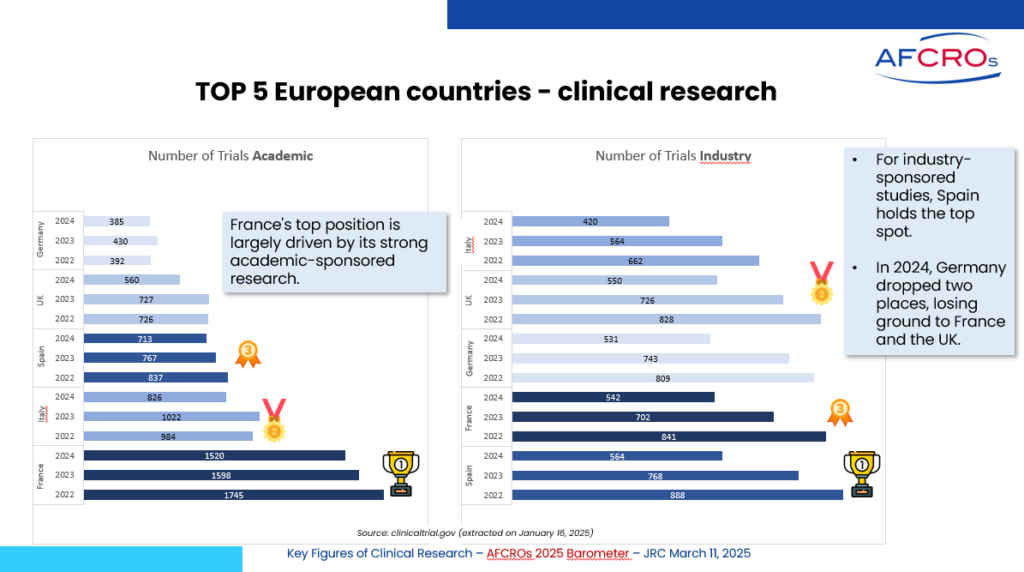
Clinical research in France - Key figures
- In 2024, clinical research activity in Europe continued to decline, a trend that has been ongoing since the end of the COVID-19 period. This decline affecting all European countries, appears to be benefiting the Asia-Pacific region and the United States. One possible explanation is the implementation of new EU regulations on drugs and medical devices, which require a longer adaptation period than initially expected, combined with increasing competition from other regions worldwide.
- France maintains its leading position in Europe, primarily due to its strong academic research activity, particularly in interventional studies. Industry-sponsored research in France has declined more sharply than academic research. The decline in medical device studies (MD) in 2024 is also notable, especially for observational studies, which are becoming less relevant under the stricter requirements of the European Medical Device Regulation and In Vitro Diagnostic Regulations (MDR & IVDR).

Patients contributions in medical product life cycle
Patient experience data is increasingly being collected and considered at every stage of the medical product lifecycle. By integrating the voice of patients, important concepts such as shared medical decision-making, patient quality of life, as well as the enhancement of care quality can be considered in the evaluation and monitoring of these products. In addition to health authority recommendations advocating for the systematic integration of patient experience in healthcare product evaluation, this data, being rich and multidimensional, can also serve as a tool for communication and differentiation in a competitive market. It also enables the regulation of the healthcare system with devices and systems that integrate indicators of the quality of the care pathway.

Reuse of RWE data in Decentralized Clinical Trials
While totally decentralized and dematerialized studies are not yet possible in France, there are solutions to get closer to this goal while we wait for the regulations to change.

Early acces pathway in France: all you want to know
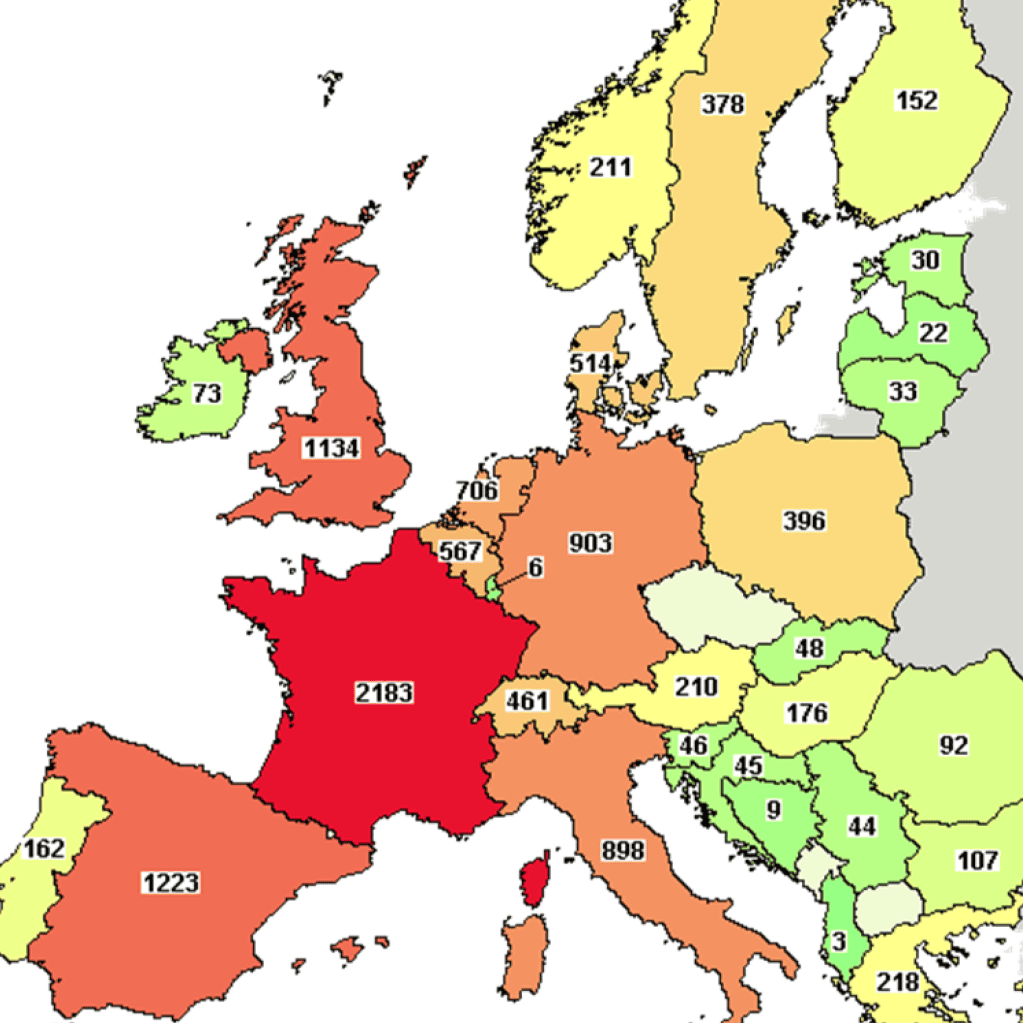
Clinical Research in France - Key figures
France remains one of the European leaders in clinical research in 2022.
Despite a slight drop in research activity in 2022, France remains positioned as a leader in the initiation of clinical studies in Europe ahead of Spain and UK. In terms of industrial trials, France is behind Spain and is almost on par with Germany.

AFCROs whitepaper | Decentralized clinical trials: from words to action
Based on feedback, interviews with various stakeholders, and the views of
different actors in the care of patients, this white paper aims to determine the
optimal conditions for carrying out a hybrid decentralized clinical study in the
home.
25 October 2022

13th July 2022
AFCROs Guide to the appropriate use of data from the National Health Data System (SNDS)
The RWD working group of AFCROs, which brings together expert consultancy firms, presents the first practical guide integrating the whole circuit of data feedback in the SNDS as well as some recommendations on the most relevant way to use them.

8th July 2021
Why France for clinical research
The association’s attractiveness group offers you a few key points on why you should choose France for your clinical trials

